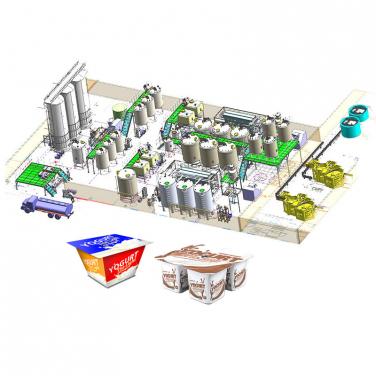
A bone peptide processing line is a series of interconnected industrial processes and equipment designed to extract and refine bioactive peptides from animal bones, typically for use in pharmaceuticals, nutraceuticals, or functional foods. Here's an overview of the main steps involved:
1. Raw Material Reception and Preparation:•Bones are sourced from slaughterhouses and undergo thorough cleaning to remove fat, meat, and other impurities.•They may be ground into smaller pieces using specialized bone crushing machines.
2. Acid or Alkali Treatment:•The bone particles are treated with hydrochloric acid or sodium hydroxide to demineralize the bone matrix, which releases collagen and peptides.
3. Enzymatic Hydrolysis:•The demineralized bone material is then subjected to enzymatic hydrolysis, often using proteolytic enzymes like pepsin or trypsin, to break down the proteins into peptides of varying lengths.
4. Neutralization and Filtration:•After hydrolysis, the mixture is neutralized to adjust pH and then filtered through various stages (e.g., centrifugation, microfiltration, ultrafiltration) to separate peptides from larger molecules and impurities.
5. Peptide Extraction and Concentration:•Further purification may involve solvent extraction or evaporation under vacuum to concentrate the peptide solution.
6. Ion Exchange Chromatography or Membrane Separation:•To obtain specific peptides with desired properties, advanced separation techniques such as ion exchange chromatography or membrane filtration are used.
7. Drying and Granulation:•The purified peptides are dried, often using spray drying technology, to form a powder that can be easily handled and formulated into different products. This powder might also go through granulation if required.
8. Quality Control and Testing:•Throughout the process, samples are collected and analyzed to ensure purity, composition, and activity of the extracted peptides.
9. Packaging and Storage:•Once the bone peptides meet quality standards, they are packaged in suitable containers, sealed, and stored under controlled conditions until ready for use or distribution.
10. Sterilization and Sanitation:•All equipment is designed to operate under hygienic conditions and many incorporate CIP (Clean-in-Place) systems for efficient cleaning and sterilization between batches.The entire bone peptide processing line requires precise control over temperature, pH, and pressure to optimize yield and ensure product safety and efficacy.
Additionally, compliance with food and pharmaceutical manufacturing regulations is crucial at every stage.

A bone peptide processing line is a sophisticated manufacturing system that extracts and refines bioactive peptides from animal bones, converting them into value-added products for use in various industries such as pharmaceuticals, nutraceuticals, cosmeceuticals, and functional foods. The technology behind this process involves several interconnected steps:
1. Raw Material Preparation:•Bones are cleaned, sanitized, and then ground or pulverized to expose the inner collagen matrix.
2. Decalcification/Defatting:•Acid or alkali treatment (such as hydrochloric acid or sodium hydroxide) is used to remove calcium and other minerals from the bone, followed by defatting using solvents or heat processes to separate fats and lipids.
3. Enzymatic Hydrolysis:•Demineralized bone material undergoes enzymatic digestion using proteolytic enzymes like pepsin, trypsin, or alkaline protease. These enzymes cleave the protein chains into smaller peptides with specific molecular weights.
4. Ultrafiltration and Membrane Separation:•After hydrolysis, the mixture is filtered through membranes of different pore sizes to isolate peptides based on their molecular weight. This can involve ultrafiltration, microfiltration, and nanofiltration techniques.
5. Ion Exchange Chromatography:•Further purification may include ion exchange chromatography, which separates peptides based on their charge properties.
6. Reverse Osmosis or Nanofiltration:•To remove any remaining impurities, reverse osmosis or nanofiltration systems might be employed.
7. Peptide Concentration and Drying:•The purified peptides are concentrated and dried, often via rotary vacuum evaporation or spray drying, to obtain a powdered form.
8. Quality Control and Analysis:•Throughout the entire process, quality control measures are implemented, including testing for peptide purity, amino acid composition, molecular weight distribution, and biological activity.
9. Sterilization and Sanitation:•Equipment is designed with CIP (Clean-in-Place) and SIP (Sterilize-in-Place) capabilities to maintain high levels of hygiene and prevent contamination.
10. Packaging and Storage:•Once processed, the bone peptides are packaged under sterile conditions, typically in hermetically sealed containers, and stored under controlled temperature and humidity to preserve their integrity.The technology in bone peptide processing lines emphasizes precision, efficiency, and safety, integrating advanced automation, data monitoring, and real-time analysis tools to optimize yields and ensure product consistency.
Additionally, compliance with GMP (Good Manufacturing Practice) standards is paramount throughout the entire production chain.

A bone peptide processing line requires a series of specialized equipment and machinery to efficiently extract, purify, and refine bioactive peptides from animal bones. Here's an overview of some essential equipment used in the process:
1. Bone Crushing Machine: To begin with, raw bones are crushed into smaller pieces using a bone crusher or grinder.
2. Cleaning and De-Fatting System: The crushed bones go through a cleaning and de-fatting stage where they are washed and treated to remove fats, impurities, and adhering meat.
3. Demineralization Equipment: This could include acid tanks for hydrolysis with acids like HCl to dissolve minerals, followed by neutralization with alkaline solutions.
4. Enzymatic Hydrolysis Reactor: A controlled enzymatic digestion process takes place here, using proteolytic enzymes such as pepsin, trypsin, or alcalase to break down proteins into peptides.
5. Filtration Systems:•Centrifuges separate larger particles from the liquid phase.•Microfiltration Units remove insoluble particulates and large molecules.•Ultrafiltration/Nanofiltration Membrane Systems fractionate peptides based on molecular weight.
6. Ion Exchange Columns: For further purification, the filtrate may pass through ion exchange columns to isolate specific peptides based on their charge properties.
7. Evaporation Systems: Concentration of the peptide solution can be achieved using rotary evaporators or falling film evaporators.
8. Dryers: Drying of the concentrated peptides is typically done using spray dryers or freeze-drying (lyophilization) equipment to produce a powdered form.
9. Sterilization/Cleaning-in-Place (CIP) Systems: All equipment must be designed for easy cleaning and sterilization to prevent contamination and ensure hygiene. CIP systems automate this process.
10. Quality Control Devices: Analytical instruments such as spectrophotometers, chromatography equipment (e.g., HPLC), and amino acid analyzers are used to test the purity, composition, and activity of the extracted peptides.
11. Packaging Machines: Once the peptides meet quality standards, they are packaged in appropriate containers using automatic filling and sealing machines.
The entire production line should be managed by a sophisticated control system that integrates all these processes and maintains strict control over parameters such as temperature, pH, pressure, and flow rates to ensure consistent product quality and yield.
The installation and debugging of equipment for bone peptide processing lines is a systematic and rigorous process, usually including the following key steps:
1. Equipment in place and infrastructure construction: After confirming the accuracy of the equipment list, all equipment will be transported to the designated location according to the predetermined plan The basic base of the equipment needs to be pre designed and constructed to ensure that it can be stable and level during installation.
2. Equipment assembly: • Assemble the equipment according to the drawings and technical guidance provided by the equipment manufacturer, including but not limited to crushers, cleaning equipment, enzymatic reactors, separation and filtration equipment, concentration and drying equipment, etc During the assembly process, pay attention to the sealing of the connections between various components and the accuracy of the transmission system.
3. Pipeline layout and connection: According to the process flow design, layout and connect material conveying pipelines, cooling water pipelines, steam pipelines, etc. to ensure smooth fluid transmission and no risk of leakage.
4. Electrical and automation system installation: • Install electrical automation components such as control panels, sensors, drive devices, and PLCs (programmable logic controllers), and lay and connect wires and cables Configure SCADA or DCS systems to achieve centralized monitoring and automatic control of the production line.
5. Single machine trial operation of equipment: • Complete independent testing and operation of each equipment, check whether all performance indicators meet the design requirements, such as crushing effect, mixing speed, temperature control accuracy, etc.
6. Linkage debugging and optimization: • Conduct linkage debugging of the entire production line, check the coordination between various equipment and the coherence of the overall process flow Adjust equipment parameters and optimize process flow through actual operational data to achieve optimal production efficiency and product quality.
7. Cleaning, disinfection and verification: Thoroughly clean and disinfect the entire production line, and verify the CIP (online cleaning) and SIP (online sterilization) systems to ensure compliance with food safety and hygiene standards.
8. Performance acceptance and personnel training: After completing all debugging, invite relevant professional institutions to conduct performance acceptance to confirm that the equipment meets the design and usage requirements Organize operators to receive training on equipment operation, maintenance, and emergency response to ensure their ability to correctly use and manage bone peptide processing lines.
In summary, the installation and debugging of equipment on the bone peptide processing line is a work involving multiple professional techniques, which requires strict adherence to safety regulations and quality management systems to ensure efficient and stable operation of the production line and the production of high-quality products.
The final product of the bone peptide processing line is bone peptide powder or bone peptide extract, which is a protein hydrolysis product with biological activity and nutritional value. Bone peptide is a functional food additive or pharmaceutical raw material extracted from animal bones through modern biotechnology.
Specifically, after a series of processes such as fragmentation, defatting, acid hydrolysis (or enzymatic hydrolysis), separation and purification, concentration and drying, the collagen and non collagen parts extracted from bones are decomposed into bone peptides in the form of polypeptide chains. These bone peptides contain various amino acid components, especially amino acids that are beneficial to the human body such as glycine, proline, and hydroxyproline. They can be widely used in the following fields:
1. Nutritional supplements: As a dietary supplement, bone peptides can provide the amino acids needed by the human body, promote bone health, and enhance immunity.
2. Pharmaceutical preparations: In the field of medicine, bone peptides can be used to manufacture drugs and have a certain auxiliary effect on the treatment of diseases such as fracture healing, osteoporosis, and arthritis. 3. Functional foods: added to dairy products, beverages, baked goods, candies, and other foods to enhance the nutritional value and health benefits of the products.
In summary, the final product produced by the bone peptide processing line is a functional ingredient that has a positive impact on human health, and its application prospects are broad.
Shanghai Beyond Machinery Co., Ltd
Beyond Machinery specializes in the design and manufacturing of Bone peptide processing line. Please contact us now, and our professional technical engineers will customize the equipment plan for Bone peptide processing line and provide a quotation. Please contact us now to obtain the latest equipment plan and quotation.